You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Oral appliances are used to treat a serious medical condition. As such, their indication is specified by guidelines issued by the American Academy of Sleep Medicine (AASM) and the American Academy of Dental Sleep Medicine (AADSM). Parameters for the use of oral appliances in the treatment of sleep disordered breathing (SDB) were first established in 1995, and re-issued in 2005 and 2015. The parameters were based on an extensive review of the literature at the time of their adoption.
The 2005 Practice Parameters state that oral appliances are indicated for patients with snoring only, with mild-to-moderate Obstructive Sleep Apnea (OSA), and with severe OSA who cannot tolerate Continuous Positive Airway Pressure (CPAP) or for whom CPAP is contraindicated.1,2 Moreover, both diagnosis of SDB and follow-up evaluation of the effectiveness of oral appliance therapy for OSA were recognized as roles of a qualified physician. Evaluation of a patient’s dentition, periodontium, oral, and musculoskeletal health to assess the patient’s candidacy for oral appliance therapy, the subsequent fabrication and treatment with an oral appliance, adjustment of the appliance, and periodic recall (6 months, 1 year, and yearly thereafter) were recognized roles of a qualified dentist.
In accordance with these 2005 Parameters, all patients (even those with snoring only) require a diagnosis by a physician and a referral to the dentist for oral appliance therapy when OSA is diagnosed. Both the AASM and AADSM further advocate that the physician be board-certified in sleep medicine and that the dentist have documented training in dental sleep medicine. Training is available through nonprofit and for-profit commercial entities. The AADSM is the largest nonprofit organization in the US providing commercial product bias-free training to dentists in sleep medicine.3
The recently adopted 2015 Clinical Practice Guideline differs from the 2005 Practice Parameters in advocating a wider use of Mandibular Advancement Devices (MADs), including patients with snoring only, and in patients with severe OSA who are likely to be more compliant with oral appliance therapy than with CPAP.4 The Guideline also recommends use of custom-fabricated MADs over those that are prefabricated (“boil and bite”) and stresses the importance of patients with OSA undergoing a follow-up sleep study during which the efficacy of the oral appliance is evaluated. It is also suggested that the appliance be adjusted during or after the sleep study if needed to improve its efficacy in eliminating respiratory events.
Types of Appliances
All MADs are Class II medical devices and require FDA 510(k) clearance to be marketed. Some prefabricated devices can be purchased directly online by patients and fit to their teeth at home. These devices historically have been bulky and poorly retentive with no measure of control over the amount of jaw advancement.5 They run the risk of under- or overtreating a patient, and without the supervision of a dentist, can result in dental side effects that exceed those of well-fitting, custom-fabricated devices monitored by a dentist. However, some newer prefabricated devices are less bulky, more retentive, and provide a lower-cost option for treatment, often as a temporary device (Figure 1). Whether these newer prefabricated devices are as efficacious as custom-fabricated MADs and are worn the same amount of time has not been investigated.
Custom-fabricated MADs are made in the dental laboratory following protocols mandated by the companies that own the intellectual property for their designs. For most of these devices, the dental laboratory must be licensed to provide a particular type of appliance and the technicians may be required to undergo specialized training to assure company standards are maintained for their named appliances. For some designs (eg, TAP® appliance, tapintosleep.com), more than 100 laboratories have been licensed; for other designs (eg, SUAD™ appliance, SomnoMed, somnomed.com; and Narval CC™ appliance, ResMed, resmed.com), all appliances are made by a limited number of dental laboratories.
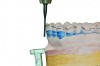
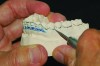
MADs typically engage both the upper and lower dentition. Most are made of either all hard acrylic or dual laminate material.6 In the former case, retention can be aided by the addition of ball clasps. These, however, can result in the development of diastemas when placed in embrasures between the teeth. For this reason, the laboratory should aim to fabricate an all-acrylic appliance that has a tight fit with the teeth, yet not engage deep undercuts that will make full seating or removal difficult and require multiple time-consuming adjustments during the appliance delivery appointment. Proper block-out and duplication of casts prior to fabrication are paramount to both the uncomplicated seating of an appliance at its delivery and good retention when worn (Figure 2 and Figure 3).
Some custom-fabricated MADs are nonadjustable, consisting of a “monoblock” design that holds the jaw forward in one fixed position. If the position does not improve the patient’s sleep respiration, a new bite registration must be taken by the dentist in a more advanced position and the device must be returned to the laboratory and rebuilt in this new position. In some cases, this process can be accomplished chairside.
Most custom-fabricated MADs prescribed today are adjustable, enabling the forward position of the mandible with respect to the maxilla to be easily adjusted to optimize efficacy of therapy in reducing respiratory events. The appliances usually have an upper and lower “tray” component (duo-block). If the appliance is designed to keep the mouth closed during sleep, each component must fit the teeth tightly; otherwise, the trays will provide little resistance to mouth opening and will lift off the teeth. Numerous designs for advancing the jaw have been patented. Most appliances advance the mandible using one of the four mechanisms described in Table 1 and Figure 4. Adjustable expansion screws, straps, bands, and midline hooks are used depending on the appliance type.
Side Effects of Therapy Short-term
Upon first use of a MAD, patients may experience short-term side effects such as an increase in salivation, soreness of some teeth, irritation of the gingiva, and mild-to-moderate masticatory muscle or TMJ discomfort.7 Of these, discomfort in the masticatory muscles or TMJ is of most concern, but it usually subsides within a few weeks or less. The process can be expedited with the application of heat, massage of the affected area, and over-the-counter pain medications (NSAIDs). Exercises to stretch the masticatory muscles in the morning and evening have also been advocated to reduce the likelihood of temporomandibular pain.8,9 Not all patients experience short-term side effects.
Long-term
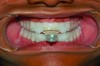
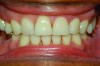
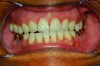
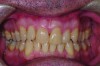
In holding the jaw forward, MADs apply backward forces to the maxillary teeth and forward forces to the mandibular teeth in proportion to the amount of advancement. With time, these forces can produce changes in tooth or jaw position, resulting in changes in the dental occlusion.10,11 A decrease in overjet and overbite is most commonly observed (Figure 5 and Figure 6).
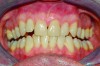
Within the first few months of treatment, some patients report difficulty achieving a firm bite with the back teeth. The mechanism underlying the development of this posterior open bite is unclear but of intense interest. When observed early after the beginning of treatment, it may be due to changes in the TMJ soft tissue or in the masticatory muscles. Later in treatment, the development of a posterior open bite may reflect the inability to fully close due to premature contact of the anterior teeth (Figure 7 and Figure 8).11 Alternatively, some investigators have suggested an anterior bony repositioning of the mandible from its original position.12
It is not possible to predict which patients will develop dental side effects or to what extent. Dental changes should be anticipated as long as the MAD is used. Morning exercises to reposition the jaw may delay their development (see Part IV).13
References
1. Kushida CA, Littner MR, Hirshkowitz M, et al. Practice parameters for the use of continuous and bilevel positive airway pressure devices to treat adult patients with sleep-related breathing disorders. Sleep. Mar 2006;29(3):375-380.
2. Ferguson KA, Cartwright R, Rogers R, Schmidt-Nowara W. Oral appliances for snoring and obstructive sleep apnea: a review. Sleep. Feb 2006;29(2):244-262.
3. Bennett K. Connecting and collaborating to advance dental sleep. Journal of Dental Sleep Medicine. 2014;1(3):111-113.
4. Ramar K, Dort LC, Katz SG, et al. Clinical Practice Guideline for the Treatment of Obstructive Sleep Apnea and Snoring with Oral Appliance Therapy: An Update for 2015. J Clin Sleep Med. Jun 11 2015.
5. Vanderveken OM, Devolder A, Marklund M, et al. Comparison of a custom-made and a thermoplastic oral appliance for the treatment of mild sleep apnea. Am J Respir Crit Care Med. Jul 15 2008;178(2):197-202.
6. Roy S. 6 Oral Appliance Material Options. Sleep Review Magazine. 2015(June):18-22.
7. Hammond RJ, Gotsopoulos H, Shen G, Petocz P, Cistulli PA, Darendeliler MA. A follow-up study of dental and skeletal changes associated with mandibular advancement splint use in obstructive sleep apnea. Am J Orthod Dentofacial Orthop. Dec 2007;132(6):806-814.
8. Ueda H, Almeida FR, Chen H, Lowe AA. Effect of 2 jaw exercises on occlusal function in patients with obstructive sleep apnea during oral appliance therapy: a randomized controlled trial. Am J Orthod Dentofacial Orthop. Apr 2009;135(4):430 e431-437; discussion 430-431.
9. Cunali PA, Almeida FR, Santos CD, et al. Mandibular exercises improve mandibular advancement device therapy for obstructive sleep apnea. Sleep Breath. Dec 2011;15(4):717-727.
10. Marklund M, Franklin KA, Persson M. Orthodontic side-effects of mandibular advancement devices during treatment of snoring and sleep apnoea. Eur J Orthod. Apr 2001;23(2):135-144.
11. Pliska BT, Nam H, Chen H, Lowe AA, Almeida FR. Obstructive sleep apnea and mandibular advancement splints: occlusal effects and progression of changes associated with a decade of treatment. J Clin Sleep Med. 2014;10(12):1285-1291.
12. Bondemark L. Does 2 years' nocturnal treatment with a mandibular advancement splint in adult patients with snoring and OSAS cause a change in the posture of the mandible? Am J Orthod Dentofacial Orthop. Dec 1999;116(6):621-628.
13. Dort LC. “Getting over” occlusal changes. Journal of Dental Sleep Medicine. 2015;2(1):3.
About the Authors
Gregory K. Essick, DDS, PhD
Professor
Department of Prosthodontics and Center for Pain Research and Innovation
School of Dentistry
University of North Carolina
Chapel Hill, NC
Andrew R. Blank, AAS, BS
Dental Student
East Carolina University School of Dental Medicine
Greenville, NC
Jamison R. Spencer, DMD, MS
Director
The Center for Sleep Apnea and TMJ
Boise, ID
Jonathan A. Parker, DDS
Owner
Clinical Director
Snoring and Sleep Apnea Dental Treatment Center
Edina, MN