You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Sleep problems in our society are very prevalent. National Sleep Foundation surveys show that 75% of Americans report at least one sleep symptom and approximately 60% of adults drive while drowsy each year.1 Sixty-seven percent of adults snore. This condition affects the quality of their sleep and the sleep of others near them. Obstructive sleep apnea (OSA) is one of the most prevalent sleep disorders, and it affects 26% of adults between the ages of 30 and 70.2 People with untreated OSA can experience a significantly reduced quality of life. It can lead to difficulty in daytime functioning due to daytime sleepiness, fatigue, irritability, and decreased cognitive function. In addition, research studies show that people with OSA have a higher risk of cardiovascular disease (hypertension, heart attack, stroke, atrial fibrillation, etc.), diabetes, asthma, cancer, and dementia.3,4 People with this condition are also more likely to have motor vehicle accidents, which affects all of us.5,6
Pathophysiology
Anatomical and neuromuscular factors underlie the pathophysiology of OSA.7 The thin-walled upper airway is surrounded by a soft-tissue mass that is bounded by the bony mandible anteriorally, and the spine posteriorally.8 Within the soft tissue mass are muscles that serve to keep the airway open. The most important of these pharyngeal dilator muscles is the genioglossus muscle, which protrudes the tongue. The patency of the airway may be compromised by a mandible that is relatively too small (micro- and/or retro-gnathia), a soft-tissue mass that is relatively too large (e.g., from a large tongue and/or cervical fat), or a dilator muscle response to airway resistance that is insufficient.9
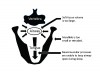
In individuals with normal sleep respiration, the activity of the pharyngeal dilator muscles is in balance with the size of the bony enclosure and soft-tissue mass, maintaining an open upper airway.7 However, in individuals with OSA, activity of the dilator muscles is insufficient to keep the airway open continuously during sleep (Figure 1).10 Periodically there is collapse of the upper airway and obstruction to airflow behind the soft palate or tongue. The collapse tends to occur at the end of expiration when effort to keep the airway open is minimal, or during inspiration when intra-airway pressure is most negative. The brain senses the blockage of airflow and arouses from sleep, leading to deeper breaths and restoration of airflow. This process of collapse-obstruction-arousal constitutes a respiratory event. Respiratory events can occur hundreds of times during a night of sleep.
The arousals of respiratory events disrupt the normal sleep pattern. Many patients fail to reach Rapid Eye Movement (REM) “dream” sleep or the deeper restorative stages of non-REM sleep.11 Rather, patients remain in lighter stages of sleep, disrupted by arousals. This sleep fragmentation is associated with many of the symptoms reported by adult patients with SDB, which differ strikingly from the effects in children (Table 1).12,13
The arousals of respiratory events not only disrupt sleep architecture but also stimulate the sympathetic nervous system, thereby elevating blood pressure and leading to hypertension over time.14 Moreover, each blockage of airflow leads to a transient drop in the oxygen saturation of hemoglobin in the blood, further stimulating the sympathetic nervous system. The intermittent hypoxia and stimulation of the sympathetic nervous system activates inflammatory processes throughout the body and promotes tissue inflammation.
Diagnosis and Classification
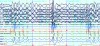
A patient with symptoms of a sleep disorder needs to see a qualified physician for a comprehensive sleep evaluation. Routine examination in the dental office may also reveal possible anatomical risk factors, which may lead the dental professional to refer the patient to a qualified physician for sleep evaluation.15,16,17 Generally, a diagnostic sleep study will be ordered for patients who report symptoms of SDB such as disruptive snoring and excessive daytime sleepiness, or who have medical conditions suggestive of impaired sleep respiration such as essential hypertension. These overnight studies have traditionally been conducted in a sleep laboratory by a trained sleep technologist who monitors the patient from an observation room. As illustrated in Figure 2, sensors measure and continuously record eye movements (LOC, ROC), EEG brain activity (F3, F4, C3, C4, O1, O2), nasal pressure airflow (PTAF2), snoring (SNORE), expired CO2, chest (CHEST) and abdominal (ABD) movements reflecting respiratory effort, limb (LAT1-LAT2) and jaw muscle (CHIN1-CHIN2) EMG activity, heart rate (PR), and oxygen saturation of the blood (OSAT).18
More recently, the use of home sleep apnea testing (HSAT, also referred to as Out of Center Sleep Testing or OCST) has been advocated by some third-party payers for patients who have symptoms of sleep-disordered breathing but are otherwise in good health. Home sleep apnea testing devices vary in complexity but collect only a few channels of data and rarely the brain activity required to definitively tell when the patient is asleep or in which stage of sleep. 17,19 Regardless of the type of sleep study, it is standard of care that a board-certified sleep physician interpret the scored results of the study when ordered for diagnostic purposes.
The sleep study data are inspected and scored for respiratory events. An apnea (A) is scored when airflow ceases for 10 or more seconds in adults (Figure 3). A hypopnea (H) is scored when airflow decreases for 10 or more seconds and other criteria are met (Table 2). The criteria established by the American Academy of Sleep Medicine — generally accepted as the authority on sleep medicine in the US — can differ from the criteria required by some third-party payers for treatment, explaining why some patients may receive a diagnosis of OSA yet may not meet criteria for insurance coverage of treatment.20,21 A respiratory effort related arousal (RERA) is scored when the patient arouses from sleep due to the increased respiratory effort to maintain normal airflow and blood oxygen levels.22
The severity of sleep-disordered breathing is defined as the frequency of apneas plus hypopneas per hour. An apnea-hypopnea index (AHI) of ≥5 events/hour is considered mild OSA; ≥15 events/hour, moderate OSA; and ≥30 events/hour, severe OSA.23 Although an AHI of <5 events>24,25 Moreover, patients with a normal AHI but with RERAs often report the same debilitating daytime symptoms of OSA, such as excessive daytime sleepiness and cognitive impairment.
Natural Course and Medical Co-morbidities
Snoring at a young age may be the first sign of sleep-disordered breathing.17 Even though the airway remains open, upper airway resistance is higher than normal, leading to turbulent airflow and vibration of the soft palate (snoring). With aging and weight gain, the resistance continues to increase, leading with time to obstructive hypopnea and apnea events.26 OSA is more prevalent in adult males than females, particularly in middle-aged males who are overweight. Although overweight middle-aged females are also prone to OSA, younger, normal-weight females may be at increased risk of milder forms of sleep-disordered breathing characterized by snoring and an increased frequency of RERAs (referred to as Upper Airway Resistance Syndrome).27 These individuals may also be at increased risk of temporomandibular disorders and fibromyalgia.28
Over time, the pathological processes associated with respiratory events (stimulation of the sympathetic nervous system, chronic intermittent hypoxia, and activation of proinflammatory pathways; see Pathophysiology section above) contribute to diseases that decrease the length and quality of life: e.g., hypertension and cardiovascular disease, cerebrovascular disease and stroke, and metabolic syndrome/diabetes (Figure 4).14 OSA contributes to these diseases above and beyond the contribution of other risk factors associated with both the diseases and OSA, obesity being recognized as the most important such factor. Untreated OSA increases risk of mortality, particularly for individuals older than 50, and efficacious treatments of OSA decrease mortality.29 Untreated OSA may also contribute to dementia in older adults or to depression mimicking signs and symptoms of dementia.30
Dental Co-morbidities
A growing body of literature since 2008 suggests that untreated sleep-disordered breathing can impact orofacial and dental health. Mounting evidence indicates an association between SDB and periodontal disease (PD), and SDB and temporomandibular disorders (TMD).28,31,32 A higher than normal prevalence of PD has been observed in individuals with SDB, and a higher prevalence of SDB in individuals with PD. The mechanism for this association is unknown but may relate to the impact on oral tissues of mouth breathing (common in patients with SDB) during sleep or to the systemic inflammation caused by both conditions.
Similarly, a higher than normal prevalence of TMD has been observed in individuals with SDB, and a higher prevalence of SDB in individuals with TMD.28,32 These studies, like those for periodontal disease, are largely observational; however, it has been demonstrated that TMD pain-free people who report symptoms of sleep-disordered breathing are more likely to develop TMD pain than people who are not at risk for SDB. The mechanisms underlying this association are unknown. SDB may increase the likelihood of pain in individuals who are otherwise susceptible to developing chronic pain. It is well known that pain also disturbs sleep; thus, the question often becomes: Is a possible sleep disorder contributing to a pain condition, or is the pain condition contributing to a sleep disorder?
An association between SDB and sleep bruxism (grinding the teeth during sleep) has been reported in the literature, the strongest evidence existing for children.33,34 Both snoring and parent-reported sleep bruxism are risk factors for OSA in children. For adults an association between SDB and laboratory-confirmed sleep bruxism has not been observed in rigorous controlled research studies, although an association is anecdotally reported by many practitioners.35,36 It is reasonable for adults with evidence of sleep bruxism to be screened by their dentists for signs and symptoms of SDB and referred to a physician for follow-up evaluation, if indicated. It has been suggested that lock-and-key patterns of attrition on the anterior teeth and mandibular tori may reflect the outcomes of bruxism associated with SDB. In children, sleep bruxism is often viewed by dental professionals as normal, and something that most children “grow out of.” However, SDB can significantly impact a child’s health and wellbeing and should be ruled out if signs and symptoms of SDB, such as snoring and bruxism, are present.
Disclosure
The authors had no disclosures to report.
References
1. National Sleep Foundation 2005 Sleep in America Poll. https://sleepfoundation.org/sites/default/files/2005_summary_of_findings.pdf. Published March 29, 2005. Accessed October 3, 2015.
2. Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006-1014.
3. Huang QR, Qin Z, Zhang S, Chow CM. Clinical patterns of obstructive sleep apnea and its comorbid conditions: a data mining approach. J Clin Sleep Med. 2008;4(6):543-550.
4. Abrams B. Hierarchy of comorbidity indicators for obstructive sleep apnea. Chest. 2010;137(6):1491-1492.
5. Hiestand D, Phillips B. Obstructive sleep apnea syndrome: assessing and managing risk in the motor vehicle operator. Curr Opin Pulm Med. Nov 2011;17(6):412-418.
6. Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: systematic review and meta-analysis. J Clin Sleep Med. 2009;5(6):573-581.
7. Tsuiki S, Isono S, Ishikawa T, Yamashiro Y, Tatsumi K, Nishino T. Anatomical balance of the upper airway and obstructive sleep apnea. Anesthesiology. 2008;108(6):1009-1015.
8. Isono S, Tanaka A, Tagaito Y, Ishikawa T, Nishino T. Influences of head positions and bite opening on collapsibility of the passive pharynx. J Appl Physiol (1985). 2004;97(1):339-346.
9. Watanabe T, Isono S, Tanaka A, Tanzawa H, Nishino T. Contribution of body habitus and craniofacial characteristics to segmental closing pressures of the passive pharynx in patients with sleep-disordered breathing. Am J Respir Crit Care Med. 2002;165(2):260-265.
10. Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol Respir Environ Exerc Physiol. 1978;44(6):931-938.
11. Fietze I, Quispe-Bravo S, Hansch T, Rottig J, Baumann G, Witt C. Arousals and sleep stages in patients with obstructive sleep apnoea syndrome: Changes under nCPAP treatment. J Sleep Res. 1997;6(2):128-133.
12. Guilleminault C, Partinen M, Quera-Salva MA, Hayes B, Dement WC, Nino-Murcia G. Determinants of daytime sleepiness in obstructive sleep apnea. Chest. 1988;94(1):32-37.
13. Capdevila OS, Kheirandish-Gozal L, Dayyat E, Gozal D. Pediatric obstructive sleep apnea: complications, management, and long-term outcomes. Proc Am Thorac Soc. 2008;5(2):274-282.
14. Arnardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AI. Molecular signatures of obstructive sleep apnea in adults: a review and perspective. Sleep. 2009;32(4):447-470.
15. Friedman M, Tanyeri H, La Rosa M, et al. Clinical predictors of obstructive sleep apnea. Laryngoscope. 1999;109(12):1901-1907.
16. Shigemoto S, Shigeta Y, Nejima J, Ogawa T, Matsuka Y, Clark GT. Diagnosis and treatment for obstructive sleep apnea: Fundamental and clinical knowledge in obstructive sleep apnea. J Prosthodont Res. 2015;59(3):161-171.
17. Gharibeh T, Mehra R. Obstructive sleep apnea syndrome: natural history, diagnosis, and emerging treatment options. Nat Sci Sleep. 2010;2:233-255.
18. Kakkar RK, Hill GK. Interpretation of the adult polysomnogram. Otolaryngol Clin North Am. Aug 2007;40(4):713-743.
19. Collop NA, Anderson WM, Boehlecke B, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 15 2007;3(7):737-747.
20. Berry RB, Budhiraja R, Gottlieb DJ, et al. Rules for scoring respiratory events in sleep: update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2012;8(5):597-619.
21. Thomas RJ, Guilleminault C, Ayappa I, Rapoport DM. Scoring respiratory events in sleep medicine: who is the driver--biology or medical insurance? J Clin Sleep Med. 2014;10(11):1245-1247.
22. Guilleminault C, Stoohs R, Clerk A, Cetel M, Maistros P. A cause of excessive daytime sleepiness. The upper airway resistance syndrome. Chest. 1993;104(3):781-787.
23. Epstein LJ, Kristo D, Strollo PJ, Jr., et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5(3):263-276.
24. Asker M, Asker S, Kucuk U, Kucuk HO. An overlooked cause of resistant hypertension: upper airway resistance syndrome - preliminary results. Clinics (Sao Paulo). 2014;69(11):731-734.
25. Sanders AE, Essick GK, Beck JD, et al. Periodontitis and Sleep Disordered Breathing in the Hispanic Community Health Study/Study of Latinos. Sleep. 2014.
26. Berger G, Berger R, Oksenberg A. Progression of snoring and obstructive sleep apnoea: the role of increasing weight and time. Eur Respir J. 2009;33(2):338-345.
27. Bao G, Guilleminault C. Upper airway resistance syndrome--one decade later. Curr Opin Pulm Med. 2004;10(6):461-467.
28. Dubrovsky B, Raphael KG, Lavigne GJ, et al. Polysomnographic investigation of sleep and respiratory parameters in women with temporomandibular pain disorders. J Clin Sleep Med. 2014;10(2):195-201.
29. Marshall NS, Wong KK, Liu PY, Cullen SR, Knuiman MW, Grunstein RR. Sleep apnea as an independent risk factor for all-cause mortality: the Busselton Health Study. Sleep. 2008;31(8):1079-1085.
30. Ancoli-Israel S, Palmer BW, Cooke JR, et al. Cognitive effects of treating obstructive sleep apnea in Alzheimer's disease: a randomized controlled study. J Am Geriatr Soc. 2008;56(11):2076-2081.
31. Al-Jewair TS, Al-Jasser R, Almas K. Periodontitis and obstructive sleep apnea's bidirectional relationship: a systematic review and meta-analysis. Sleep Breath. 2015.
32. Sanders AE, Essick GK, Fillingim R, et al. Sleep apnea symptoms and risk of temporomandibular disorder: OPPERA cohort. J Dent Res. 2013;92(7 Suppl):70S-77S.
33. Carra MC, Bruni O, Huynh N. Topical review: sleep bruxism, headaches, and sleep-disordered breathing in children and adolescents. J Orofac Pain. 2012;26(4):267-276.
34. Manfredini D, Restrepo C, Diaz-Serrano K, Winocur E, Lobbezoo F. Prevalence of sleep bruxism in children: a systematic review of the literature. J Oral Rehabil. 2013;40(8):631-642.
35. De Luca Canto G, Singh V, Gozal D, Major PW, Flores-Mir C. Sleep bruxism and sleep-disordered breathing: a systematic review. J Oral Facial Pain Headache. 2014;28(4):299-305.
36. Raphael KG, Janal MN, Sirois DA, et al. Validity of self-reported sleep bruxism among myofascial temporomandibular disorder patients and controls. J Oral Rehabil. 2015.