You must be signed in to read the rest of this article.
Registration on CDEWorld is free. You may also login to CDEWorld with your DentalAegis.com account.
Many different ceramic systems have been introduced in recent years for all types of indirect restorations from conservative no-preparation veneers to multiple-unit posterior fixed partial dentures (FPDs) and everything in between. Knowing the various nuances of materials and processing systems is overwhelming and can be confusing. Using a classification of the microstructural components of ceramics, this article covers the types of ceramics available. A second simpler classification system established on how the ceramics are processed will provide the main guidelines for their use.
The term ceramic is derived from the Greek word "keramos," which means "potter" or "pottery." This is related to a Sanskrit term meaning "burned earth," because the basic components were clays from the earth that were heated to form pottery. Ceramics are nonmetallic inorganic materials and refer to numerous materials, including metal oxides, borides, carbides, and nitrides, as well as complex mixtures of these materials.1 Their structure is crystalline, displaying a regular periodic arrangement of the component atoms, and may exhibit ionic or covalent bonding. Although ceramics can be very strong, they are also extremely brittle and will catastrophically fail after minor flexure. Thus, these materials are strong in compression but weak in tension.
Contrast that to metals, which are nonbrittle (display elastic behavior) and ductile (display plastic behavior). This is because of the nature of the interatomic bonding, which is called metallic bonds. Defining these bonds is a cloud of shared electrons that can easily move when energy is applied. This is what makes most metals great conductors. Ceramics can be very translucent to very opaque. In general, the glassier the microstructure (ie, noncrystalline), the more translucent the ceramic will appear; the more crystalline, the more opaque. Other contributory factors to translucency include particle size, particle density, refractive index, and porosity, just to name a few.
There are many clinical and technical aspects that are important for success with all-ceramic materials but are not as critical with metal-based restorations and not possible to cover here. A basic clinical use guide is shown in Table 1. The reader is advised that significant knowledge and training in these areas are requisites for technical success with all-ceramic materials.
The Different Ceramics Used in Dentistry
The term ceramic technically refers to a crystalline material. Porcelain is a mixture of glass and crystal components. A noncrystalline-containing material is simply a glass. However, dentistry typically refers to all three basic materials as dental ceramics. How ceramics are classified can be confusing. Ceramics can be divided by their microstructure (ie, amount and type of crystalline phase and glass composition), processing technique (powder/liquid, pressed, or machined), and clinical application. To provide the reader with a better understanding of ceramics, the authors give a classification based on the microstructure of ceramics, with the inclusion of how the ceramics are processed, which affects durability.
Microstructural Classification
At a microstructural level, ceramics can be defined by their composition of glass-to-crystalline ratio. There can be infinite variability of the microstructures of materials; however, they can be divided into four basic compositional categories with a few subgroups:
- Composition Category 1: Glass-based systems (mainly silica)
- Composition Category 2: Glass-based systems (mainly silica) with fillers usually crystalline (typically leucite or a different high-fusing glass)
- Composition Category 3: Crystalline-based systems with glass fillers (mainly alumina)
- Composition Category 4: Polycrystalline solids (alumina and zirconia)
Composition Category 1: Glass-based Systems, Amorphous Glass
Glass-based systems are made from materials that contain mainly silicon dioxide (also known as silica or quartz), which have various amounts of alumina. Naturally occurring aluminosilicates, which contain various quantities of potassium and sodium, are known as feldspars. Feldspars are modified in different ways to create the glasses used in dentistry. Synthetic forms of aluminosilicate glasses are also manufactured for dental ceramics. The authors found no documented references that showed naturally occurring aluminosilicate glasses perform better or worse than synthetic even though there have been claims to the contrary. These materials were first used in dentistry to make porcelain dentures.
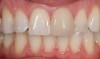
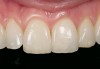
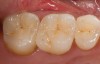
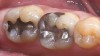
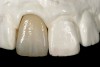
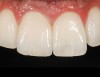
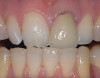
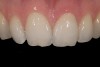
Mechanical properties are low, with flexural strength usually from 60 MPa to 70 MPa. Thus, they tend to be employed as veneer materials for metal or ceramic substructures, as well as for veneers, using either a refractory die technique or platinum foil. The microstructure of a glass is shown in Figure 1. This is a scanning electron micrograph of an acid-etched glass surface. The holes indicate a second glass, which was removed by the acid. The veneer restoration uses a glassy porcelain (Figure 2 and Figure 3).
Composition Category 2: Glass-based Systems with Crystalline Second Phase, Porcelain
This category has a large range of glass-crystalline ratios and crystal types, so much so that the authors subdivided this category into three groups. The glass composition is similar to the pure glass Category 1. The difference is varying amounts of crystal types have either been added to or grown in the glassy matrix. The primary crystal types today are either leucite, lithium disilicate, or fluorapatite. Leucite is created in dental porcelain by increasing the K2O (potassium oxide) content of the aluminosilicate glass. Lithium-disilicate crystals are made by adding Li2O (lithium oxide) to the aluminosilicate glass. It also acts a flux, lowering the melting temperature of the material.
These materials have also been developed into fine-grain machinable blocks—Vitablocs Mark II (Vident, vident.com) for use with CEREC® computer-aided design/computer-aided manufacturing (CAD/CAM) system (Sirona, www.sirona.com). Sirona CERECblocs are fabricated by Vita using the Vitablocs Mark II powders; however, Sirona has a different shade system. This material is the most successfully documented machinable glass for the fabrication of inlays and onlays with all studies showing a less than 1% per year failure rate, which compares favorably with metal-ceramic survival data.2-7 A pre-manufactured block has no residual porosity in the finished core that could act as a weak point and lead to catastrophic failure.
Subcategory 2.1
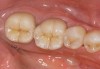
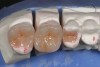
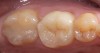
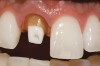
Low-to-moderate leucite-containing feldspathic glass: Even though other categories have a feldspathic-like glass, these materials have been called feldspathic porcelains by default. Leucite may alter the coefficient of thermal expansion (CTE), as well as inhibit crack propagation, thereby improving the material's strength. The amount of leucite may be adjusted in the glass based on the type of core and required CTE. These materials are the typical powder/liquid materials used to veneer core systems and are also ideal for porcelain veneers. The original materials had a fairly random size and distribution of leucite crystals with the average particle size of approximately several hundred microns. This random distribution and large particle size contributed to the material's low-fracture resistance and abrasive properties relative to enamel.8 Newer generations of materials have been developed with much finer leucite crystals (10 µm to 20 µm) and very even particle distribution throughout the glass. These materials are less abrasive and have much higher flexural strengths.9 In Figure 4, a scanning electron micrograph (SEM) of a typical feldspathic porcelain reveals a glass matrix surrounding leucite crystals. These materials are most commonly used as veneer porcelains for metal-ceramic restorations (Figure5 ).
Subcategory 2.2
High-leucite (approximately 50%)-containing glass, glass-ceramics: The microstructure of these materials consists of a glass matrix surrounding a second phase of individual crystals. The material starts as a homogeneous glass. A secondary heat treatment nucleates and grows crystals, which gives this class improved mechanical and physical properties due to the physical presence of the crystals and generation of compressive stress around the crystals. Glass-ceramics may be ideally suited for use as dental restorative materials and generally have improved mechanical and physical properties, such as increased fracture resistance, improved thermal shock resistance, and resistance to erosion. Improvements in properties depend on the interaction of the crystals and glassy matrix, as well as on the crystal size and amount. Finer crystals generally produce stronger materials. Glass-ceramics are in widespread use for cookware, missile nose cones, and even heat shields on space vehicles. They may be opaque or translucent depending on the chemical composition and percent crystallinity. A fundamental method of improving strength and fracture resistance is to add a second phase to a glass material—dispersion strengthening. The crystals may act as roadblocks to cracks. A crack growing from a defect must go through or around the crystal; this diverts some energy from the propagating crack and may stop it entirely. Thus, the restoration may continue to function instead of cracking in half. In addition to the "roadblock" effect, compressive stresses around the growing crystals may help pin cracks and further enhance fracture resistance.
The most widely used version is the original pressable ceramic Empress® (Ivoclar Vivadent, www.ivoclarvivadent.com) (Figure 6, Figure 7 and Figure 8). A number of pressable materials with properties and microstructure similar to Empress are available. These include Finesse® (DENTSPLY, www.dentsply.com), Authentic® (Jensen, www.jensendental.com), PM™9 (Vita, www.vident.com), and OPC (Pentron, www.pentronceramics.com) A machinable version Empress CAD (Ivoclar) designed for both CEREC® (Sirona, www.sirona.com) and E4D® CAD/CAM (D4D Technologies, www.e4dsky.com) systems for high-leucite ceramics has performed well clinically when used for posterior inlays and onlays, as well as anterior veneer and crown restorations.9-14 Paradigm™ C porcelain block (3M ESPE, www.3mespe.com) has a similar properties. Machinable and pressable systems have much higher fracture resistance than powder/liquid systems and have shown excellent clinical results for posterior inlay and onlay applications and anterior veneer and crown restorations.2-7,10-14
Subcategory 2.3
Lithium disilicate glass-ceramics: This is a true glass-ceramic introduced initially by Ivoclar as Empress II (and now in the form of IPS e.max® pressable and machinable ceramics). Increasing the crystal content to approximately 70% and refining the crystal size improved flexural strength. The glass matrix consists of a lithium silicate with micron-size lithium-disilicate crystals in between, which are submicron lithium-orthophosphate crystals (Figure 9, Figure 10 and Figure 11). This creates a highly filled glass matrix. A veneer porcelain consisting of fluorapatite crystals in an aluminosilicate glass may be layered on the core to create the final morphology and shade of the restoration. The shape and volume of crystals increase the flexural strength to approximately 360 MPa, or about three times that of Empress.15-19 This material can be translucent even with the high crystalline content; this is due to the relatively low refractive index of the lithium-disilicate crystals. The material is translucent enough that it can be used for full-contour restorations or for the highest esthetics and can be veneered with special porcelain. Veneer porcelain consisting of fluorapitite crystals in an aluminosilicate glass may be layered on the core to create the final morphology and shade of the restoration. Fluorapitite is a fluoride-containing calcium phosphate, Ca5(PO4)3F. The fluorapitite crystals contribute to the veneering porcelain's optical properties and CTE, so it matches the lithium-disilicate pressable or machinable material. Both the veneering and lithium-disilicate materials are etchable due to the glassy phase. Initial clinical data for single restorations are excellent with this material, especially if it is bonded.20 A material with similar properties and structure called 3G OPC is available as a pressable glass-ceramic from Pentron.
Composition Category 3: Interpenetrating Phase Ceramics
In-Ceram® (Vident, www.vident.com) consists of a family of all-ceramic restorative materials based on the same principle introduced in 1988. The family includes a range of strengths, translucencies, and fabrication methodology designed to cover the wide scope of all-ceramic restorations, including veneers, inlays, onlays, and anterior/posterior crowns and bridges. In-Ceram Spinell (alumina and magnesia matrix) is the most translucent with moderately high strength and used for anterior crowns. In-Ceram Alumina (alumina matrix) has high strength and moderate translucency and is used for anterior and posterior crowns. In-Ceram Zirconia (alumina and zirconia matrix) has very high strength and lower translucency and is used primarily for three-unit posterior bridges. In addition, these materials are supplied in a block form for producing milled restorations using a variety of machining systems.
In-Ceram is in a class called interpenetrating phase composites.21 They consist of at least two phases, which are intertwined and extend continuously from the internal to external surfaces (Figure 12). This class has better mechanical and physical properties relative to the individual components; a tortuous route through alternating layers of both components is required in order for these materials to break.
Interpenetrating phase materials are generally fabricated by first creating a porous matrix; in the case of In-Ceram, it would be a ceramic "sponge." The pores are then filled by a second-phase material, lanthanum-aluminosilicate glass, using capillary action to draw a liquid or molten glass into all the pores to produce the dense interpenetrating material.
The system was developed as an alternative to conventional metal-ceramics and has met with great clinical success.22,23 The system uses a sintered crystalline matrix of a high-modulus material (85% of the volume) in which there is a junction of the particles in the crystalline phase. This is different than glasses or glass-ceramic materials in that these ceramics consist of a glass matrix with or without a crystalline filler in which there is no junction of particles (crystals). Slip casting24 may be used to fabricate the ceramic matrix, or it can be milled from a presintered block.25 Flexural strengths range from 350 MPa for spinell, 450 MPa for alumina, and up to 650 MPa for zirconia. Several clinical studies support the use of In-Ceram Alumina for single units placed anywhere in the mouth. In-Ceram Alumina had the same survival rates as porcelain-fused-to-metal restorations up to the first molar, with a slightly higher failure rate for the second molar.26-28 In-Ceram Zirconia should only be used on molars due to its very high opacity, which is not suitable for anterior esthetics. For anterior teeth, the alumina magnesia version of In-Ceram (called Spinell) is ideal due to its higher translucency (Figure 13, Figure 14 and Figure 15).
Composition Category 4: Polycrystalline Solids
Solid-sintered monophase ceramics are formed by directly sintering crystals together without any intervening matrix to form a dense, air-free, glass-free, polycrystalline structure. Several processing techniques allow the fabrication of either solid-sintered aluminous oxide (alumina, Al2O3) or zirconium oxide (ZrO2) framework. The first fully dense polycrystalline material for dental applications was Procera® AllCeram alumina (Nobel Biocare, www.nobelbiocare.com), with a strength of approximately 600 MPa.29 The alumina powder is pressed and milled on a die and sintered at about 1600°C, leading to a dense coping but with approximately 20% shrinkage (Figure 16, Figure 17 and Figure 18).
The use of what is commonly referred to in dentistry as zirconia has increased rapidly in the past few years. This is not pure zirconia; it is partially stabilized by the addition of small amounts of other metal oxides. Partially stabilized zirconia allows production of reliable multiple-unit all-ceramic restorations for high-stress areas, such as the posterior region of the mouth. Zirconia may exist in several crystal types (phases) depending on the addition of minor components, such as calcia (CaO), magnesia (MgO), yttria (Y2O3), and ceria (CeO2). Specific phases are said be stabilized at room temperature by the minor components. Typically for dental applications, about 3 weight% of yttria is added to pure zirconia (Figure 19, Figure 20 and Figure 21).
Zirconia has unique physical characteristics that make it twice as strong and tough as alumina-based ceramics. Values for flexural strength range from approximately 900 MPa to 1100 MPa.30,31 There is no direct correlation between flexural strength (modulus of rupture) and clinical performance. Another important physical property is fracture toughness, which has been reported between 8 MPa m1/2 and 10 MPa m1/2 for zirconia.30 This is significantly higher than any previous dental ceramic. Fracture toughness is a measure of a material's ability to resist crack growth. Zirconia has the apparent physical properties to be used for multiple-unit anterior and posterior FPDs. Clinical reports on zirconia have not demonstrated a problem with the zirconia framework.32,34 The problems have been associated with chipping and cracking of porcelain. Using a slow-cooling protocol at the glaze bake to equalize the heat dissipation from zirconia and porcelain increased the fracture resistance of the porcelain by 20%. Zirconia may be in the form of porous or dense blocks that are milled to create the frameworks or recently, full-contour single-unit restorations. Most are fabricated from a porous block, milled oversized by about 25%, and sintered to full density in a 4- to 6-hour cycle. An alternate approach involves milling a fully dense block. However, due to the nature of zirconia, this approach requires approximately 2 hours of milling time per unit whereas milling of the porous block necessitates only 30 to 45 minutes for a three-unit bridge.
Within Classifications 2 and 3, compositions can vary greatly. Several commercial materials are in these groups. Glass-based systems (Category 1 and Category 2) are etchable and thus easily bondable. Crystalline-based systems (Category 3 and Category 4) are not etchable and much more difficult to bond. Categories 1 to 3 can exist in a powdered form that is then fabricated using a wet-brush technique, or they can also be preprocessed into a block that can be pressed or machined. As a general rule, powder/liquid systems have much lower strength than pre-manufactured blocks due to a much larger amount of bubbles and flaws in the finished restoration.
Classification Based on Processing Technique
A more user-friendly and a simplistic way to classify the ceramics used in dentistry is by how they are processed. All materials can be processed by varied techniques; however, in general for dentistry, they can be classified as: 1) powder/liquid glass-based systems; 2) machinable or pressable blocks of glass-based systems; and 3) CAD/CAM or slurry die-processed mostly crystalline (alumina or zirconia) systems. It is an important classification method, as there appears to be a greater correlation to clinical success (and thus failure) due to processing techniques. Even though a material may have the same chemistry and microstructure, the processing methodology used to produce a restoration may improve or decrease the final properties and clinical success. Specifically, machined blocks of materials have performed better than powder/liquid versions of the same material.
1. Powder/Liquid
1A. Conventional
These are typically veneer materials, which may be all glass or a mixture of glass and crystal components. These include veneers for all-ceramic and metal frameworks and may also be used alone as anterior veneer restorations. Typically, these materials are hand-mixed with de-ionized water or a special modeling liquid supplied by the manufacturer. They are built up by hand and vibrated (condensed) to remove water and air. These are fired in a vacuum to help remove remaining air and improve the density and esthetics of the veneer. Because these restorations are handmade, voids are often present in the fired material. This is inherent to the process and may be worse or better depending on environmental conditions, the technician's skill, and the firing cycle. Frequently, one sees bubbles remaining in the hand-layered veneer material.
1B. Slip Casting
The original In-Ceram and some partially stabilized zirconia blocks are fabricated based on slip casting of alumina or zirconia. The "slip" is a homogenous dispersion of ceramic powder in water. The water pH is often adjusted to create a charge on the ceramic particles, and the ceramic powder is coated with a polymer to cause the particles to be evenly suspended in the water. In the case of In-Ceram, the slip is "painted" on a gypsum die with a brush to form the underlying core for the ceramic tooth. The water is removed via capillary action of the porous gypsum, which packs the particles into a rigid network (Figure 22). The alumina core is then slightly sintered (0.2% shrinkage) in a furnace to create an interconnected porous network. The lanthanum glass powder is placed on the core; the glass becomes molten and flows into the pores by capillary action to produce the interpenetrating network. The last step in the fabrication involves application of aluminous porcelain on the core to produce the final form of the restoration. Other powder dispersions, such as those created with zirconia, may be poured into a gypsum mold that withdraws the water and leads to a homogeneous block of zirconia being formed.
2. Pressable
Pressed ceramic restorations are fabricated using a method similar to injection molding. Monochromatic porcelain or glass-ceramic ingots are heated to allow the material to flow under pressure into a mold formed using a conventional lost-wax technique. The restoration may be cast to its final contours and subsequently stained and glazed to provide an esthetic match. Alternatively, a coping may be molded on which porcelain is added to achieve the restoration's final shape and shade. Empress restorations and other materials with a similar leucite/glass structure are fabricated in this manner. The glass-ceramic IPS e.max is also created this way. Pressables may be used for inlays, onlays, veneers, and single-unit crowns.
3. CAD/CAM
3A. Subtractive
Full-contour
Full-contour restorations such as inlays, onlays, crowns, and veneers may be fabricated from various blocks of materials. In general, these blocks are fabricated from starting powders that are mixed with a binder and then pressed into a mold or extruded like a sausage into a block form. The binder helps hold the powder together so that the shape is maintained after pressing or extrusion. Then, the blocks are transferred to a furnace to remove the binder and sinter to full density. As mentioned previously, restorations milled from blocks tend to have improved density and mechanical properties as compared with powder/liquid or pressed restorations due to the standardized manufacturing process (Figure 23).35,36
Glass/Crystal
Vitablocs are fabricated using fine-grain powders, producing a nearly pore-free ceramic with fine crystals. This was the first material specifically produced for the CEREC system and has an excellent history of clinical success for inlays, onlays, and anterior and posterior crowns.36 Sirona CERECblocs are fabricated from the same powders. The restoration may be characterized with external stains, or porcelain may also be added to produce a layered effect (Figure 24 and Figure 25). These blocks are available as monochromatic, polychromatic with stacked shades as in a layer cake, and in a form replicating the hand-fabricated crowns whereas an enamel porcelain is layered on top of dentin porcelain.
Glass/Leucite
Empress CAD is based on the pressable Empress and has the same microstructure—a feldspathic glass with approximately 45% leucite crystal component. These blocks also have a fine leucite crystal structure (approximately 5 µm to 10 µm) and may also be further characterized using external stains or porcelain. Empress CAD is available in monochromatic and polychromatic stacked shades. Strength properties are similar to Vitablocs. A common theme to all of these blocks is a fine particle-size microstructure that helps resist machining damage, improve mechanical properties, and decrease polishing time of the finished restoration.
Lithium Disilicate
The IPS e.max® block (lithium disilicate) is not initially fully crystallized, which improves milling time and decreases chipping risk from milling. The milled restoration is then heat-treated for 20 to 30 minutes to crystallize the glass and produce the final shade and mechanical properties of the restoration. This crystallization changes the restoration from blue to a tooth shade. The microstructural and chemical composition is essentially the same as IPS e.max® Press. The e.max block has several translucencies, the least translucent being used primarily as a framework material and the higher translucency blocks used for full-contour restorations.
Framework Alumina: Interpenetrating Phase/Glass-Infused In-Ceram blocks are fabricated by pressing the alumina-based powder into a block shape similar to Vitablocs. However, these blocks are only fired to approximately 75% density. Porous blocks of In-Ceram materials are milled to produce a framework. The blocks are then infused with a glass in different shades to produce a 100% dense material, which is then veneered with porcelain. Glass infusion only requires 20 minutes for a coping and 1.5 hours for a three-unit bridge. The microstructure is the same as the slip cast alumina. The blocks are available in all three types of In-Ceram.
Alumina:
Porous Alumina frameworks may be fabricated from porous blocks of material. Pressing the alumina powder with a binder into molds produces the blocks. The blocks may be partially sintered to improve resistance to machining damage or used as pressed in a fully "green" state (unfired, with binder). The frameworks are milled from the blocks and then sintered to full density at approximately 1500°C for 4 to 6 hours. The alumina has a fine particle size of about 1µm and strength of approximately 600 MPa and is designed for anterior and posterior single units, as well as anterior three-unit bridges.
Partially Stabilized Zirconia:
Porous Zirconia frameworks milled from porous blocks are fabricated similarly to alumina blocks. There are various methods to press the powder into a mold. Uni-axial involves pressing from one direction, biaxial means pressing from two equal and opposite directions, and isostatic essentially indicates uniform pressing in all directions. All methods have advantages and disadvantages; however, the desired result is the same, that is, to produce a homogeneous block that shrinks uniformly. As is the case with the alumina block, the milled zirconia framework shrinks about 25% after a 4- to 6-hour cycle at approximately 1300°C to 1500°C. The particle size is about 0.1 µm to 0.5 µm.
Partially Stabilized Zirconia:
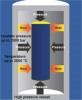
"HIP" blocks Fully dense zirconia is produced by hot isostatic pressing. The zirconia powder may be prepressed in a block, or the powder itself is packed into a flexible mold. Either the blocks or mold is then vacuum-sealed in an airtight rubber or plastic bag and placed into a fluid-filled chamber. Pressure is then applied to the fluid and transmitted evenly around the zirconia. Heat is applied to the chamber, which sinters the zirconia to full density (Figure 26). Zirconia blocks produced in this manner may achieve flexural strength values of approximately 1200 MPa to 1400 MPa. However, it requires extended milling to produce the framework, and the higher strength value does not generally justify the lost productivity. The accuracy may be improved versus the porous block method and may be preferred for large frameworks that span the arch.
3B. Additive
Electrodeposition
In-Ceram powder dispersions used in the slip casting technique have been applied to electrodeposition systems, which apply a current across the dispersion and deposit the powder particles automatically on the surface of a conductive die. This approach is efficient for single units but becomes cumbersome and potentially unreliable for multiple-unit frameworks.
Discussion and Summary
Ceramics can be classified in many ways. Two classification systems were given to aid the reader in understanding the types of ceramics available for dental use. Processing technique has a large impact on strength and thus clinical performance and should be one of the primary considerations in choosing a material. There are many clinical aspects that are important for success with all-ceramic materials but are not as critical with metal-based restorations and not possible to cover here (eg, preparation design, management of stresses, cementation techniques). A basic clinical usage guide is shown in Table 1. The reader is advised that significant knowledge and training in these areas are requisites for success with all-ceramic materials.
References
1. Kingery WD, Bowen HK, Uhlmann DR. Introduction to Ceramics. 2nd ed. New York, NY: John Wiley and Sons;1976:1-19.
2. Otto T. CEREC restorations. CEREC inlays and onlays: the clinical results and experiences after 6 years of use in private practice [in French, German]. Schweiz Monatsschr Zahnmed. 1995;105(8):1038-1046.
3. Reiss B, Walther W. Überlebensanalyse und klinische Nachuntersuchungen von zahnfarbenen Einlagefüllungen nach dem CEREC-Verfahren [in German]. Zahnaerztl Welt. 1992;100(5):329-332.
4. Heymann HO, Bayne SC, Sturdevant JR, et al. The clinical performance of CAD-CAM-generated ceramic inlays: a four-year study. J Am Dent Assoc. 1996;127(8):1171-1181.
5. Berg NG, Derand T. A 5-year evaluation of ceramic inlays (CEREC). Swed Dent J. 1997;21(4):121-127.
6. Reiss B, Walther W. Ereignisanalyse und klinische Langzeitergebnisse mit Cerec-Keramikinlays [in German]. Dtsch Zahnarztl Z. 1998;53(1):65-68.
7. Reiss B, Walther W. Clinical long-term results and 10-year Kaplan-Meier analysis of Cerec restorations. Int J Comput Dent. 2000;3(1):9-23.
8. McLaren EA, Giordano RA, Pober R, et al. Material testing and layering techniques of a new two phase all glass veneering porcelain for bonded porcelain and high alumina frameworks. Quintessence Dent Technol. 2003;26:69-81.
9. McLaren EA, Giordano RA. Zirconia-based ceramics: material properties, esthetics, and layering techniques of a new veneering porcelain, VM9. Quintessence Dent Technol. 2005;28:99-111.
10. Wagner J, Hiller KA, Schmalz G. Long-term clinical performance and longevity of gold alloy vs ceramic partial crowns. Clin Oral Investig. 2003;7(2):80-85.
11. Brochu JF, El-Mowafy O. Longevity and clinical performance of IPS-Empress ceramic restorations–a literature review. J Can Dent Assoc. 2002;68(4):233-237.
12. Kraemer N, Frankenberger R. Clinical performance of bonded leucite-reinforced glass ceramic inlays and onlays after 8 years. Dent Mater. 2005;21(3):262-271.
13. Manhart J, Chen HY, Neuerer P, et al. Three-year clinical evaluation of composite and ceramic inlays. Am J Dent. 2001;14(2):95-99.
14. van Dijken JW, Hasselrot L, Ormin A, et al. Restorations with extensive dentin/enamel-bonded ceramic coverage. A 5-year follow-up. Eur J Oral Sci. 2001;109(4):222-229.
15. Albakry M, Guazzato M, Swain MV. Fracture toughness and hardness evaluation of three pressable all-ceramic dental materials. J Dent. 2003;31(3):181-188.
16. Albakry M, Guazzato M, Swain MV. Biaxial flexural strength, elastic moduli, and x-ray diffraction characterization of three pressable all-ceramic material. J Prosthet Dent. 2003;89(4):374-380.
17. Guazzato M, Albakry M, Ringer SP, et al. Strength, fracture toughness and microstructure of a selection of all-ceramic materials. Part I. Pressable and alumina glass-infiltrated ceramics. Dent Mater. 2004;20(5):441-448.
18. Hoeland W, Schweiger M, Frank M, et al. A comparison of the microstructure and properties of the IPS Empress 2 and the IPS Empress glass ceramics. J Biomed Mater Res. 2000;53(4):297-303.
19. Della Bona A, Mecholsky JJ Jr, Anusavice KJ. Fracture behavior of Lithia disilicate and leucite based ceramics. Dent Mater. 2004;20(10):956-962.
20. Piwowarczyk A, Lauer HC, Sorensen JA. In vitro shear bond strength of cementing agents to fixed prosthodontic restorative materials. J Prosthet Dent. 2004;92(3):265-273.
21. Clarke D. Interpenetrating phase composites. J Am Ceram Soc. 1992;75:739-759.
22. Proebster L. Survival rate of In-Ceram restorations. Int J Prosthodont. 1993;6(3):259-263.
23. Scotti R, Catapano S, D'Elia A. A clinical evaluation of In-Ceram crowns. Int J Prosthodont. 1995;8(4):320-323.
24. Proebster L, Diehl J. Slip casting alumina ceramics for crown and bridge restorations. Quintessence Int. 1992;23(1):25-31.
25. McLaren EA, Sorensen JA. High strength alumina crown and bridge substructures generated using copy milling technology. Quintessence Dent Technol. 1995;18:31-38.
26. Seghi RR, Daher T, Caputo A. Relative flexural strength of dental restorative ceramics. Dent Mater. 1990;6(3):181-184.
27. Giordano R, Pelletier L, Campbell S, et al. Flexural strength of alumina and glass components of In-Ceram. J Dent Res. 1992;71:253.
28. McLaren EA, White SN. Survival of In-Ceram crowns in a private practice: a prospective clinical trial. J Pros Dent. 2000;83(2):216-222.
29. Hegenbarth EA. Procera aluminum oxide ceramics: a new way to achieve stability, precision, and esthetics in all-ceramic restorations. Quintessence Dent Technol. 1996;20:21-34.
30. Piwowarczyk A, Ottl P, Lauer HC, et al. A clinical report and overview of scientific studies and clinical procedures conducted on the 3M ESPE Lava All-Ceramic System. J Prosthodont. 2005;14(1):39-45.
31. Papanagiotou HP, Morgano SM, Giordano RA, et al. In vitro evaluation of low-temperature aging effects and finishing procedures on the flexural strength and structural stability of Y-TZP dental ceramics. J Prosthet Dent. 2006;96(3):154-164.
32. Sailer I, Feher A, Filser F, et al. Five year clinical results of zirconia frameworks for posterior fixed partial dentures. Int J Prosthodont. 2007;20(4):383-388.
33. Christensen RP, Eriksson KA, Ploeger BJ. Clinical performance of PFM, zirconia, and alumina three-unit posterior prostheses [abstract]. http://iadr.confex.com/iadr/2008Toronto/techprogram/abstract_105962.htm. Accessed June 6, 2010.
34. Raigrodski AJ, Chiche GJ, Potiket N, et al. The efficacy of posterior three-unit zirconium-oxide-based ceramic fixed partial dental prostheses: A prospective clinical pilot study. J Prosthet Dent. 2006;96(4):237-244.
35. Stappert CF, Guess PC, Chitmongkolsuk S, et al. All-ceramic partial coverage restorations on natural molars. Masticatory fatigue loading and fracture resistance. Am J Dent. 2007;20(1):21-26.